
PHYsics 1151
Homework
Chapter 16; Temperature and Heat

|
ToC, Chapter 16 | Course
Calendar |

D16.1 If you have a fever of 102° on a Fahrenheit thermometer,
what is your temperature on a Celsius thermometer?
TC = [5/9]
[TF - 32°F ]
TC = [5/9] [102°F -
32°F ]
TC = [5/9] [70 F°
]
TC = [5 C°/9 F°]
[70 F° ]
TC = 39°C
D16.2 On a concrete road, how large should the expansion joints be between
sections if each section is 15 m long. Consider a temperature range of -10°C
to + 35°C.
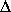 l
=
l
= 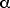 lo
lo 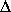 T
T
From the text, we find the coefficient of thermal expansion for concrete to
be
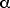 = 12 x 10-6 (C°)-1
= 12 x 10-6 (C°)-1
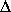 l
= [12 x 10-6 (C°)-1] [15 m] [45
C°]
l
= [12 x 10-6 (C°)-1] [15 m] [45
C°]
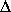 l
= 8.1 x 10-3 m
l
= 8.1 x 10-3 m
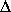 l
= 8.1 mm
l
= 8.1 mm
D16.3 A steel surveying tape is carefully calibrated at 20°C. However,
it is used on a 38°C summer day. Are the distances measured too large or
too small? What is the percentage of error introduced by this temperature change?
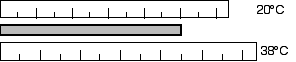
Due to the increase in temperature, the tape will elongate or get
longer. The length measured with the longer surveying tape will be
too small.
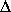 l
=
l
= 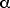 lo
lo 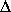 T
T
From Table 12.1, p 430, we find the coefficient of thermal
expansion for steel to be
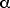 = 12 x 10-6 (C°)-1
= 12 x 10-6 (C°)-1
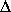 l
= [12 x 10-6 (C°)-1] [ lo]
[18 C°]
l
= [12 x 10-6 (C°)-1] [ lo]
[18 C°]
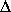 l
= 2.16 x 10-4 lo = 2.2 x 10-4 lo
l
= 2.16 x 10-4 lo = 2.2 x 10-4 lo
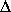 l
/ lo = .0022 = 0.22%
l
/ lo = .0022 = 0.22%
(not a very big change, of course!)
D16.4 A home furnace is rated at 50,000 Btu/h. What is this power rating
in kilowatts?
The conversion from Btu to J is .
P = 50,000 Btu/h = 50,000
Btu/h [1054.8
J/Btu][h/3600 s] =
1.465 x 104 J/s
P = 1.465 x 104 J/s = 14.65 x
103 J/s
[W/(J/s)] = 14.65 x 103 W =
14.67 kW
D16.5. A child’s wading pool contains 1.2 m3 of water at
15°C. How much heat must be added to the pool to bring the temperature to
27°C?
We can find the heat needed -- but in terms of the mass of the water. What
is the mass of 1.2 m3 of water?
m =  V = (1000 kg / m3) (1.2 m3) = 1.2 x 10 3
kg = 1,200 kg
V = (1000 kg / m3) (1.2 m3) = 1.2 x 10 3
kg = 1,200 kg
 Q
= c m
Q
= c m  T
T
 Q
= [ 4186 J / kg C° ] [ 1,200 kg ]
[ 12 C° ]
Q
= [ 4186 J / kg C° ] [ 1,200 kg ]
[ 12 C° ]
 Q
=6.03 x 107 J
Q
=6.03 x 107 J
D16.6 Once its melting point is reached, how much heat is required to melt
a 0.5 kg bar of gold?
From the text, the Heat of Fusion for gold is Lf = 0.644 x 105
J/kg
Q = Lf m
Q = [ 0.644 x 105 J / kg ] [ 0.5
kg ] = 0.322 x 105 J = 3.22 x 104 J = 32,200 J

|
ToC, Chapter 16 | Course
Calendar |

(c)2005, Doug Davis; all rights reserved


